Chemical engineers from Indiana University in the U.S. have created a molecule that utilises solar energy to convert atmospheric carbon dioxide into greenhouse-neutral fuels, which can then be stored or used for power generation.
The molecule works by using light or electricity to trigger a reaction that converts carbon dioxide (CO2) into carbon monoxide, a carbon-neutral fuel source. Because burning fuels releases CO2 and energy, physical chemistry dictates that reconverting it back into a neutral fuel source requires at least the same amount of energy.
Achieving this reconversion in high enough efficiencies has become a major goal among climate scientists, as it could lead to scrubbing CO2 – one of the leading causes of global warming – from the Earth’s atmosphere.
“If you can create an efficient enough molecule for this reaction, it will produce energy that is free and storable in the form of fuels,” said team leader Liang-shi Li (picutured), associate professor in the IU Bloomington College of Arts and Sciences’ Department of Chemistry. “This study is a major leap in that direction.”
Described as a nanographene-rhenium complex, the molecule uses nanographene – nanometres-thick segments of graphite. It’s an effective solar collector due to its dense black colour and ability to absorb a larger portion of the solar spectrum.
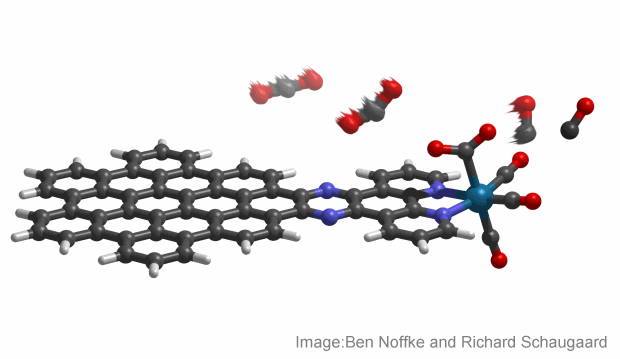
Combined with an organic compound known as bipyridine, when exposed to sunlight the nanographene absorbs solar energy and triggers a flow of electrons to the rhenium, which in turn repeatedly binds and converts the normally stable carbon dioxide to carbon monoxide.
“Carbon monoxide is an important raw material in a lot of industrial processes,” Li said. “It’s also a way to store energy as a carbon-neutral fuel since you’re not putting any more carbon back into the atmosphere than you already removed. You’re simply re-releasing the solar power you used to make it.”
The process, published in the Journal of the American Chemical Society, requires the least amount of energy to drive the formation of carbon monoxide reported thus far.
Li and his team will now work enhancing the efficacy of the molecule, ramping up the conversion power, and making it more durable in a solid state. They also intend replacing the rhenium atom, a rare and expensive element, with manganese, lowering the cost of the system.